2024
Shifts in receptors during submergence of an encephalitic arbovirus.
Li W*, Plante JA, Lin C, Basu H, Plung JS, Fan X, Boeckers JM, Oros J, Buck TK, Anekal PV, Hanson WA, Varnum H, Wells A, Mann CJ, Tjang LV, Yang P, Reyna RA, Mitchell BM, Shinde DP, Walker JL, Choi SY, Brusic V, Llopis PM, Weaver SC, Umemori H, Chiu IM, Plante KS, Abraham J.
Nature. 2024. doi: https://doi.org/10.1038/s41586-024-07740-2.
Structural and functional analysis of the Nipah virus polymerase complex.
Hu S*, Kim H*, Yang P*, Yu Z, Ludeke B, Mobilia S, Pan J, Stratton M, Bian Y, Fearns R**, Abraham J**.
bioRxiv. 2024. doi: https://doi.org/10.1101/2024.05.29.596445.
2023
Structural basis for VLDLR recognition by eastern equine encephalitis virus.
Yang P*, Li W*, Fan X, Pan J, Mann CJ, Varnum H, Clark LE, Clark SA, Coscia A, Nabel Smith K, Brusic V, Abraham J.
bioRxiv. 2023. doi: https://doi.org/10.1101/2023.11.14.567065.
2022
VLDLR and ApoER2 are receptors for multiple alphaviruses.
Clark LE*, Clark SA*, Lin CY*, Liu J*, Coscia AC, Nabel KG, Yang P, Neel DV, Lee H, Brusic V, Stryapunina I, Plante KS, Ahmed AA, Catteruccia F, Young-Pearse TL, Chiu IM, Llopis PM, Weaver SC, Abraham J.
Nature. 2022. 602: 475–480.
Structural basis for continued antibody evasion by the SARS-CoV-2 receptor-binding domain.
Nabel KG*, Clark S.A.*, Shankar S*, Pan J*, Clark L.E., Yang P, Coscia A, McKay L.G.A., Varnum HH, Brusic V, Tolan NV, Zhou G, Desjardins M, Turbett SE, Kanjilal S, Sherman AC, Dighe A, LaRocque RC, Ryan ET, Tylek C, Cohen-Solal JF, Darcy AT, Clabers A, Fan Y, Griffiths A, Correia IR, Seagal J, Baden LR, Charles RC, Abraham J.
Science. 2022. DOI: 10.1126/science.abl6251.
Emerging enterococcus pore-forming toxins with MHC/HLA-I as receptors.
Xiong X*, Tian S*, Yang P*, Lebreton F, Bao H, Sheng K, Yin L, Chen P, Zhang J, Qi W, Ruan J, Wu H, Chen H, Breault DT, Wu H, Earl AM, Gilmore MS**, Abraham J**, Dong M**.
Cell. 2022. 185, 1157–1171
Monoclonal antibodies with extended half-life to prevent COVID-19.
Abraham J.
New England Journal of Medicine. 2022. 386:2236-2238 DOI: 10.1056/NEJMe2205563 (Editorial)
Host receptor-targeted therapeutic approach to counter pathogenic New World mammarenavirus infections.
Hickerson BT, Daniels-Wells TR, Payes C, Clark LE, Candelaria PV, Bailey KW, Sefing EJ, Zink S, Ziegenbein J, Abraham J, Helguera G, Penichet ML, Gowen BB.
Nature Communications. 2022. 13(1), 558.
FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation.
Junqueira C, Crespo Â, Ranjbar S, de Lacerda LB, Lewandrowski M, Ingber J, Parry B, Ravid S, Clark S, Schrimpf MR, Ho F, Beakes C, Margolin J, Russell N, Kays K, Boucau J, Das Adhikari U, Vora SM, Leger V, Gehrke L, Henderson LA, Janssen E, Kwon D, Sander C, Abraham J, Goldberg MB, Wu H, Mehta G, Bell S, Goldfeld AE, Filbin MR, Lieberman J.
Nature. 2022. 606, 576–584.
IgM antibodies derived from memory B cells are potent cross-variant neutralizers of SARS-CoV-2.
Hale M, Netland J, Chen Y, Thouvenel CD, Smith KN, Rich LM, Vanderwall ER, Miranda MC, Eggenberger J, Hao L, Watson MJ, Mundorff CC, Rodda LB, King NP, Guttman M, Gale M, Abraham J, Debley JS, Pepper M, Rawlings DJ.
JEM. 2022. 219(9):e20220849.
Antibody-based inhibition of pathogenic New World hemorrhagic fever mammarenaviruses by steric occlusion of the human transferrin receptor 1 apical domain.
Ferrero S, Flores MD, Short C, Vazquez CA, Clark LE, Ziegenbein J, Zink S, Fuentes D, Payes C, Batto MV, Collazo M, García CC, Abraham J, Cordo SM, Rodriguez JA, Helguera G.
Journal of Virology. 2022. 95(17), e0186820.
2021
SARS-CoV-2 evolution in an immunocompromised host reveals shared neutralization escape mechanisms.
Clark SA*, Clark LE*, Pan J*, Coscia A, McKay LGA, Shankar S, Johnson RI, Brusic V, Choudhary MC, Regan J, Li JZ, Griffiths A, Abraham J. (2021)
Cell. 2021. 184 (10):2605-2617.e18.
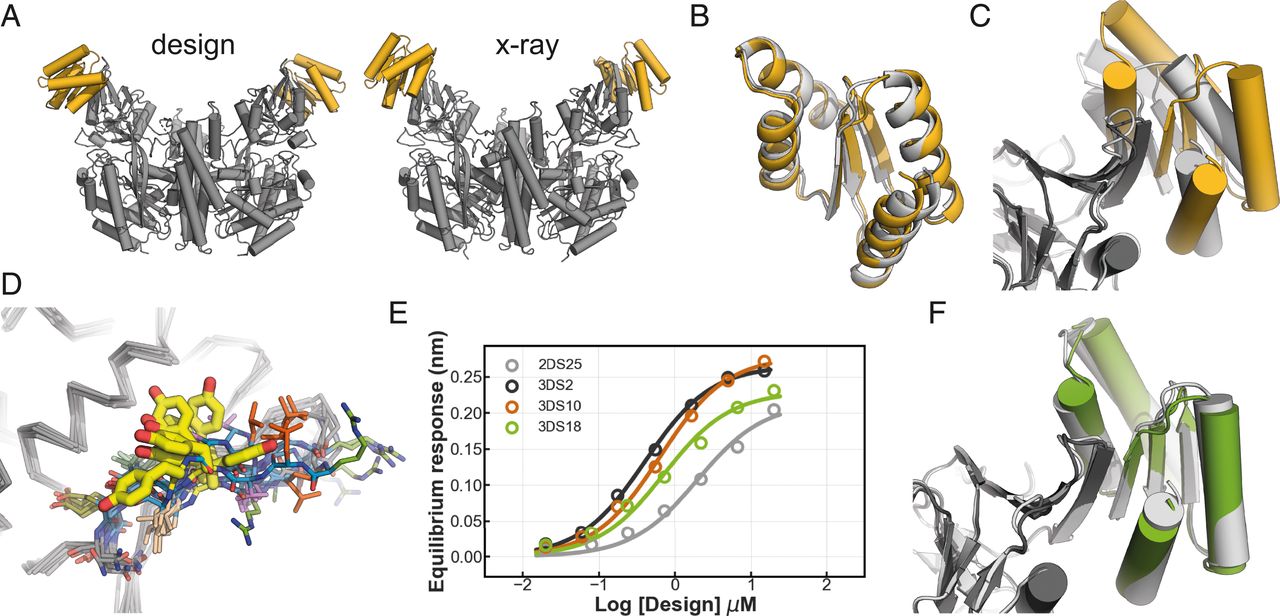
Transferrin receptor targeting by de novo sheet extension.
Sahtoe DD, Coscia A, Mustafaoglu N, Miller LM, Olal D, Vulovic I, Yu TY, Goreshnik I, Lin YR, Clark L, Busch F, Steward L, Wysocki VH, Ingber DE, Abraham J**, Baker D.**
PNAS. 2021.
Glycoprotein N-linked glycans play a critical role in arenavirus pathogenicity.
Koma T, Huang C, Coscia A, Hallam S, Manning JT, Maruyama J, Walker AG, Miller M, Smith JN, Patterson M, Abraham J, Paessler S.
PLoS Pathogens 2021. (3):e1009356.
Rapid generation of potent antibodies by autonomous hypermutation in yeast.
Wellner A, McMahon C, Gilman MSA, Clements JR, Clark S, Nguyen KM, Ho MH, Hu VJ, Shin JE, Feldman J, Hauser BM, Caradonna TM, Wingler LM, Schmidt AG, Marks DS, Abraham J, Kruse AC, Liu CC.
Nature Chemical Biology. 2021. 17(10), 1057-1064.
2020
Passive antibody therapy in COVID-19.
Abraham J.*
Nature Reviews Immunology. 2020. 20, 401-403.
2018
Vaccine-elicited receptor-binding site antibodies neutralize two New World hemorrhagic fever arenaviruses.
Clark LE, Mahmutovic S, Raymond DD, Dilanyan T, Koma T, Manning JT, Shankar S, Levis SC, Briggiler AM, Enria DA, Wucherpfennig KW, Paessler S, Abraham J*.
Nature Communications. 2018. 9,1884.
Transferrin receptor 1 is a reticulocyte-specific receptor for Plasmodium vivax.
Gruszczyk J, Kanjee U, Chan LJ, Menant S, Malleret B, Lim NTY, Schmidt CQ, Mok YF, Lin KM, Pearson RD, Rangel G, Smith BJ, Call MJ, Weekes MP, Griffin MDW, Murphy JM, Abraham J, Sriprawat K, Menezes MJ, Ferreira MU, Russell B, Renia L, Duraisingh MT, Tham WH.
Science. 2018. 359 (6371):48-55.
2015
Molecular basis for antibody-mediated neutralization of New World hemorrhagic fever mammarenaviruses.
Mahmutovic S, Clark L, Levis SC, Briggiler AM, Enria DA, Harrison SC, Abraham J.
Cell Host & Microbe. 2015. 18, 1-9.
Prior Publications
2010
Structural basis for receptor recognition by New World hemorrhagic fever arenaviruses.
Abraham J, Corbett KD, Farzan M, Choe H, Harrison SC. (2010)
Nature Structural and Molecular Biology. 17(4):438-444.
2009
Host-species transferrin receptor 1 orthologs are cellular receptors for nonpathogenic New World clade B arena viruses.
Abraham J*, Kwong JA*, Albariño CG, Lu JG, Radoshitzky SR, Salazar-Bravo J, Farzan M, Spiropoulou CF, Choe H.
PLoS Pathogens. 5(4): e1000358.